What Is The PH Scale for Acids?
Unleash Your Inner Chemist: Decoding the Mysterious PH Scale!
Curious about what Is the PH scale for acids? Explore this comprehensive guide to understand acidity levels and their impact on everyday life. The pH scale is utilized to determine if a substance is acidic or alkaline. It is widely used in many scientific and industrial applications, ranging from the food industry to medical research. The scale ranges from 0-14, with 7 being neutral, below 7 being acidic, and above 7 being basic or alkaline.
Understanding the pH scale is important for a variety of reasons, such as controlling the acidity of soil or water for gardening or fish keeping; maintaining water quality for swimming pools; and ensuring that products like cosmetics are safe for use on skin. This article will explore what exactly the pH scale measures and why it is so important.
Definition of the pH Scale
The pH scale gauges the level of acidity or alkalinity in a solution and has a range of 0 to 14. 7 is the midpoint of neutrality. Solutions with pH values below 7 are considered acidic, while those with values above 7 are considered alkaline.
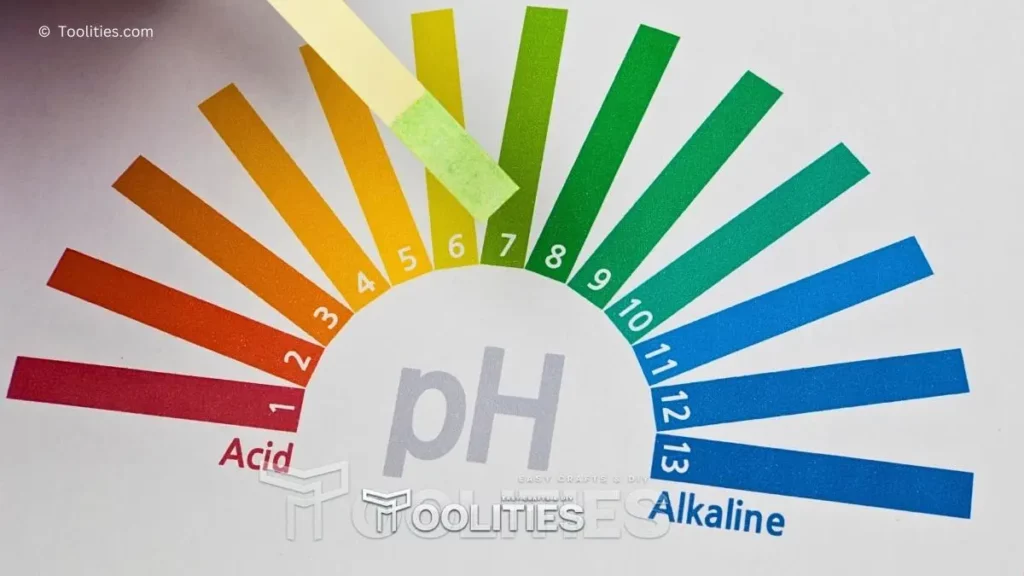
The lower the number on the scale, the more acidic the solution is, and vice versa. A pH of 0 is very acidic while a pH of 14 is very alkaline. An important thing to remember about the pH scale is that each unit represents a 10-fold difference in acidity or alkalinity; for instance, a solution with a pH of 4 is 10 times more acidic than one with a pH of 5.
Additionally, most living organisms prefer to live in environments with neutral pH levels, so it's important for scientists to monitor and maintain proper pH levels when conducting experiments involving living organisms.
Overview of Acids and their properties
Acids are a type of chemical substance that have properties that allow them to react with other substances in order to produce new compounds. Acids typically contain hydrogen ions and are capable of donating protons or accepting electrons, which causes them to be highly reactive. Acids can be categorized by strength, with strong acids having the most concentrated hydrogen ion concentration and weak acids having less hydronium ions.
Generally, acids have a sour taste and have a pH lower than 7 on the pH scale. They also tend to react with bases to form salts and water, though some types of acids are able to react with metals as well as organic molecules. As such, acids play an important role in both home-based activities and industrial manufacturing processes.
What is the pH Scale for Acids?
The pH scale for acids is a measure of the relative acidity or alkalinity of a solution. The scale varies from 0 to 14, where the midpoint is 7 and represents neutrality. Values below 7 indicate an acidic solution, and values above 7 indicate an alkaline/basic solution. The lower the pH value, the more acidic the solution is; conversely, the higher the pH value, the more basic it is.
Acids generally have a pH below 7, whereas bases have a pH above 7. The most common acids found in nature are hydrochloric acid (HCl), sulfuric acid (H2SO4), and nitric acid (HNO3). These acids have a very low pH value, usually between 0 and 3. Bases like sodium hydroxide (NaOH) or potassium hydroxide (KOH) have higher pH values, usually between 11 and 14.
The Basics of the pH Scale
The pH scale is a measure of acidity or alkalinity that is used in many fields, including chemistry, biology and medicine. The pH scale spans from 0 to 14 where a reading of 7 denotes neutrality. Substances that have a pH reading below 7 are acidic and those above it are alkaline. Substances with a low pH are more acidic while substances with a high pH are more alkaline.
Acids generally have a sour taste while bases or alkalis generally have a bitter taste. The pH scale can also be used to measure the strength of an acid or base, with higher numbers indicating a stronger acid or base. Knowing the basics of the pH scale can help you understand the chemistry and reactions between different acids and bases.
Measuring Acidity Levels with the pH Scale
The pH scale is a measure of acidity or basicity, ranging from 0 to 14. A pH of 7 is considered neutral, with values below 7 being acidic and above 7 being basic. Measuring the acidity levels of a solution can be done using the pH scale. To begin, the solution needs to be tested with a liquid that changes color depending on its acidity level. This liquid is known as an indicator and is typically litmus paper or a universal indicator solution.
Once the indicator has been placed in the solution and left for several minutes, it will change color to indicate its pH level. The color can then be compared against a chart to determine the exact pH level of the solution. With this method, it is easy to measure how acidic or basic a substance is and adjust accordingly.
Interpreting Results from the pH Scale
Interpreting results from the pH scale is essential to understanding how acidic or basic a substance is. The pH range spans from zero to 14, where zero denotes maximum acidity and 14 implies maximum alkalinity. Any number below 7 signifies acidity, whereas any number above 7 signifies basicity.
A neutral pH of 7 indicates that there are equal amounts of hydrogen and hydroxide ions in the substance. It's important to note that even small changes in pH can have significant effects on a substance. For example, a change from 6 to 5 on the pH scale could indicate a tenfold increase in acidity. Therefore, it is important to accurately interpret results from the pH scale in order to make accurate observations about a substance and its properties.
Applications of the pH Scale for Acids
The pH scale is used to measure the acidity or alkalinity of a solution. It ranges from 0 to 14, with 7 being neutral. Solutions with a pH less than 7 are acidic and those with a pH greater than 7 are alkaline. The applications of the pH scale for acids is vast, ranging from food and beverage production to medical science and environmental studies.
In food and beverage production, the acidity of ingredients such as vinegar or lemon juice can be measured using this scale. In medical science, it is used in blood tests to measure acid levels in the body as well as in urine tests to detect urinary tract infections. In environmental studies, it is used to measure acid
Testing for Acidity Levels in Solutions / Alkalinity
Testing the acidity levels in a solution is an important process for many industries. This can be done using a variety of methods, such as litmus paper, pH meters, and titration. Litmus paper is the simplest way to test for acidity levels, as it will change color when exposed to different pH levels. A pH meter is more accurate and can measure the exact level of acidity in a solution.
Titration is a process that uses a burette and reagent to measure the amount of acid in a sample. This method is most often used by scientists and chemists when precise measurements are needed. All three methods provide useful information about the acidity levels of a solution and can help identify potential problems.
Adjusting the Acidity Levels in Solutions
Adjusting the acidity levels in solutions is an important process that must be done carefully. The pH scale measures the acidity or alkalinity of a substance with a range from 0 to 14, with lower numbers indicating higher acidity and higher numbers indicating higher alkalinity. Solutions can be adjusted by adding either an acid or a base, depending on the desired outcome.

Acids are substances that contain hydrogen ions, such as vinegar and lemon juice, while bases are substances that contain hydroxide ions, such as baking soda and ammonia. Adjusting the acidity levels in solutions is a delicate process that must be done accurately to ensure optimal performance of the solution. Knowing the composition of the solution and its desired outcomes will help determine what type of adjustment needs to be made to achieve these goals.
Monitoring Acidic Conditions in Environments
Monitoring acidic conditions in the environment is important to ensure the safety and health of people, animals, and plants. Acidic conditions can be caused by pollution from industrial activities, such as burning fossil fuels or chemicals used in manufacturing processes. It can also be caused by natural events like volcanic eruptions and wildfires. Monitoring acidity levels helps us understand how our environment is affected by human activities, natural disasters, and other factors.
This information can help us determine appropriate steps to take to mitigate or reduce the impact on our environment. In addition to monitoring pH levels, scientists may look at other indicators such as sulfur dioxide gas concentrations or nitrogen dioxide concentrations to get a better understanding of the environmental acidity level. Taking these measures will help create a safer and healthier environment for everyone.
Final Thoughts
The acidity or alkalinity of a solution can be determined using the pH scale, which covers a range of 0 to 14. A pH value of 7 is considered neutral, whereas a lower pH value signifies an acidic solution and a higher pH value indicates an alkaline solution.
Acids are substances that donate hydrogen ions to a solution and have a pH below 7. Bases are substances that accept hydrogen ions and have a pH above 7. The strength of acids and bases can be determined by the concentration of hydrogen ions in the solution and how far away it is from the neutral point on the scale. For example, a strong acid has a low pH value close to 0, while a weak acid has a higher pH value closer to 7.
In short, strong bases have high values close to 14, while weak bases have lower values closer to 7. Understanding how acids and bases interact with one another is important for many applications in science and industry such as medicine, food production, agriculture, and environmental conservation.






